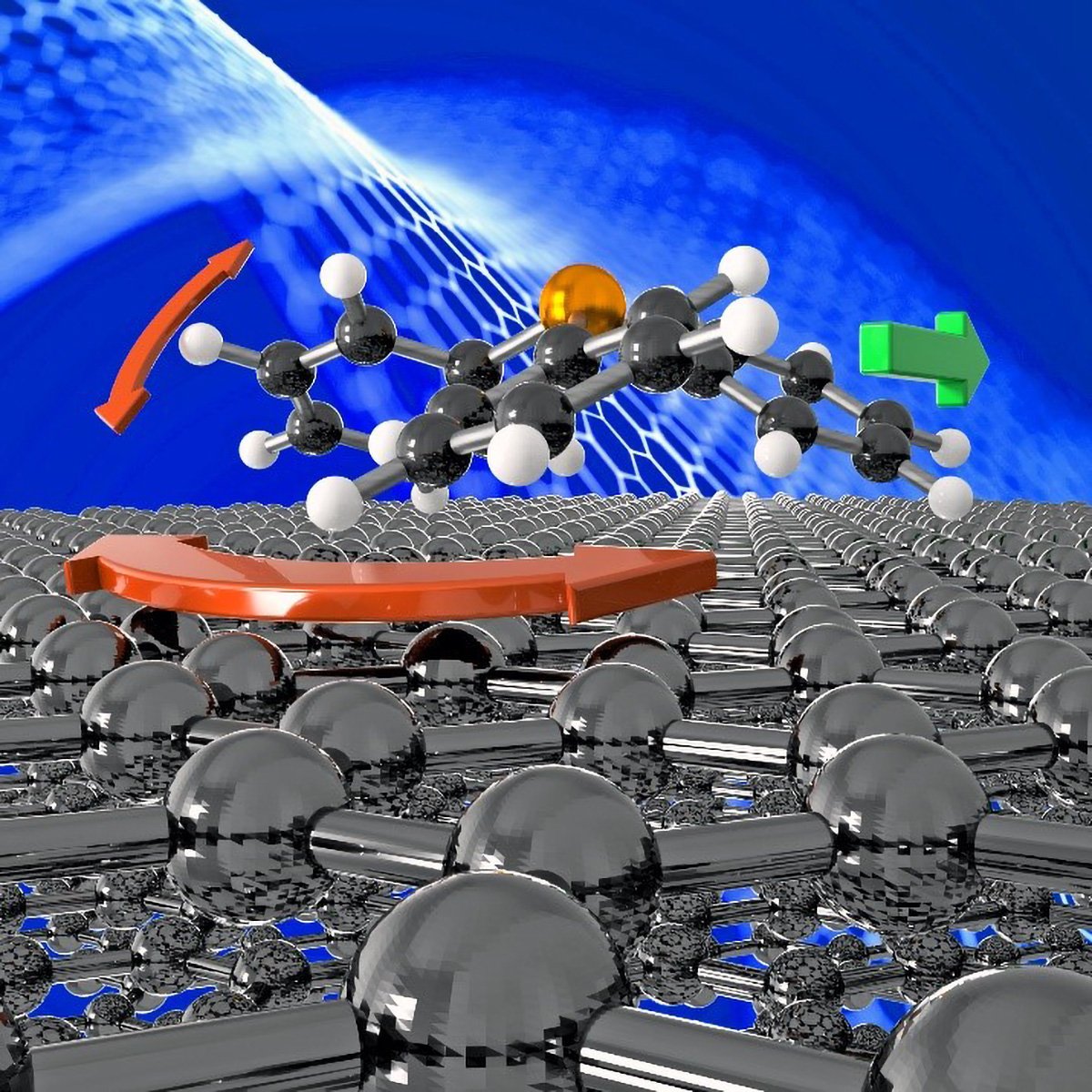
The molecule PPh3 exhibits a peculiar geometry, with a propeller-like arrangement of its three cyclic groups of atoms. This geometry, combined with its three-point binding with the surface, seems to facilitate the moonlander-like motion.
Peculiar Geometry and Binding
The molecule PPh3, which stands for triphenylphosphine, is known for its unique spatial arrangement. Its three phenyl (Ph) groups are arranged in a propeller-like configuration, giving the molecule an intricate and distinctive geometry.
Moonlander-like Motion
This specific geometry, along with the three-point binding of the molecule to the surface, appears to enable a peculiar moonlander-like motion. The propeller-like structure and the three-point attachment seem to work in tandem, facilitating a unique and fascinating mode of movement for the PPh3 molecule.